A Doctor’s Dilemma
A pediatric resident struggles with the ethics of returning genetic results to a pregnant patient
Chapter 1
Jimmy Bennett glared at his colleagues as a pager beeped for the third time, interrupting his mentor’s weekly lab meeting. Someone answer that! he thought, before realizing the sound was coming from his own bag. Rushing to silence the noise, he stared down at the number. Children’s Hospital. This was the call he had been waiting for, but it was a day late.
The young, curly-haired doctor quietly closed the door behind him and walked to the landing outside the conference room. Leaning on the banister overlooking the modern wooden foyer, he briefly thought back over the last few months, took a deep breath, and then dialed the number.

Image Courtesy of Highways Agency via Flickr
“Hello, Doug speaking.”
Doug was the chair of the hospital’s Institutional Review Board (IRB). An IRB is an ethics body; it reviews and approves all proposed research within its jurisdiction. This ensures that investigators adhere to federal research regulations. Jimmy had spoken repeatedly with Doug over the past months because he was seeking the board’s approval to disclose the results of one of his studies—perhaps the most important piece of research he’d ever done.
In the course of researching the cause of a life-threatening birth defect, Jimmy had uncovered information about one of his subjects that could be a matter of life or death. He wanted to tell the subject—his patient—but needed IRB approval to do so. He was waiting for the decision.
Doug was calling from the weekly meeting the IRB staff held to resolve complicated cases; they were discussing Jimmy’s request. He had one important question for the enthusiastic researcher:
“We need to know what the patient will do with the information. Why would you disclose?”
Chapter 2
At the center of this story is a pregnant woman—let’s call her Jane. Jane was both a patient and a research subject to Jimmy. He had first met her three years earlier, shortly after she gave birth for the first time. Her baby was born with a rare hereditary condition called congenital diaphragmatic hernia (CDH). It lived for only two months.

Pregnancy
CDH is a malformation of the diaphragm that can cause the intestines to move toward the lungs, which, in turn, causes difficulty breathing and other complications. It is often fatal in infants; it’s a condition so severe that some parents would consider terminating an affected fetus. In fact, six months later, when an eighteen-week ultrasound revealed evidence of CDH, Jane and her husband made the difficult decision to terminate their second pregnancy.
Since then, however, Jimmy had gathered strong evidence that a mutation he had recently identified was the cause of CDH in Jane’s family. Now, Jane was fourteen weeks into her third pregnancy, and if Jimmy was right about the mutation, it would be possible to screen her unborn child for the condition immediately, weeks before imaging by ultrasound or amniocentesis could be performed. But Jane didn’t know there was a genetic result, a mutation that could affect her reproductive choices.
In a clinical setting, a doctor has an obligation to act in the best interests of his patient. Researchers, however, do not typically have the same obligation, and for a variety of reasons, research results are not usually returned to subjects. Many of these reasons have to do with protecting patients; ethically speaking, if results were returned, subjects might feel coerced to participate in a research study for personal gain—to get information about themselves rather than to advance scientific knowledge. More practically, research results are not held to the same quality standards as clinical tests, and so simple mistakes such as sample mix-ups are theoretically more likely; this is not necessarily a problem within the context of a study with a large sample group, where inconsistent data can be excluded, but could be devastating to an individual. But perhaps the biggest reason research results are not typically returned is that it costs a lot of time and money to return genetic information to people.
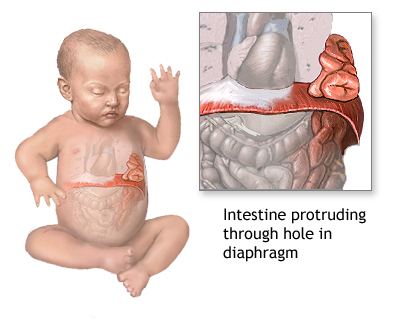
Diagram showing congenital diaphragmatic hernia (CDH) in an infant
In Jane’s case, at the most basic level, there was a technical hurdle: the consent form Jane had originally signed when Jimmy took her samples did not include any provision for returning results. Jimmy had asked the IRB to consider a modification to the original consent procedures that would allow him to tell Jane what he had learned—even though he didn’t actually know whether she would want to know.
Jimmy had spent a long time thinking about that dilemma—whether he should tell Jane. It was a tough decision. He sought advice from his colleagues, who were split on the question. His senior mentor said, “You are a doctor; you are compelled to tell her.” His attending physician said he shouldn’t disclose the research result because it wasn’t a validated clinical test, conducted in a clinical lab. Other colleagues were concerned about the risk of terminating a healthy fetus. Still others were worried the patient would sue if Jimmy withheld the information.
As he had with many hard choices in his career, Jimmy turned to his father, who is also a doctor. The two-hour time difference between the West Coast and his hometown of New Orleans meant he often called home while walking to the bus in the early evening. His dad reminded him of the Jimmy-Robbie rule, on which he had often relied during his own long medical career. That is, what would he do if it were his kids, Jimmy and Robbie? Would he want to be told or not?
The fact of the matter was that if his child were the patient, Jimmy knew he’d be pissed if his doctor didn’t disclose the result. So, when Doug asked, “Why would you disclose?” Jimmy was ready.
On countless occasions, Jimmy had explained to his colleagues how disclosing this information to Jane would grant her time—time to end her pregnancy sooner or time to prepare mentally for what lay ahead. No more questions and answers. Now, on the phone with Doug, he laid out his argument one last time. Doug promised that the IRB would have a decision in the next few hours.
Jimmy had hoped the decision would come sooner. Even as Doug and Jimmy were speaking, Jane was at the other end of the medical campus having a prenatal visit. She didn’t know that a group of researchers, ethicists, and members of the community were meeting to discuss her case. Jane lived far from the hospital; her appointments required a long trip. Jimmy had already spoken with Jane’s OB/GYN team and hoped to be able to speak with Jane at this appointment. But it wasn’t to be. As the call ended, Jimmy hoped that the next time his pager beeped, he would be relieved of his mental anguish. Before putting his phone in his pocket, he checked the time. It was mid-morning; Jane’s appointment was almost over. She would remain in the dark for at least another day.
Chapter 3
Doctors must make decisions about patient care.
Stories about researchers’ efforts to identify the causes of rare genetic disorders using whole genome sequencing are common in today’s news. Whole genome sequencing—where scientists “read” or decode almost all of a person’s DNA—offers us more information every year, and the volume of genetic data will only continue to increase. We stand to benefit tremendously from this data, but the way we use it—and how available we make it—can have significant and far-reaching impacts on individuals. Some of the risks, which have been discussed widely in the medical field as well as in newspapers and popular magazines, include breaches of privacy, genetic discrimination, and changed self-perception.
Less commonly discussed is the impact of this information on doctors and researchers. The decisions they face in light of this new technology ignite tensions between patient autonomy and medical paternalism. What happens when a doctor has a result like this? What pressures is he under? How can he take his patient’s preferences into account? Should he tell, or not?
Chapter 4
When Jimmy first met Jane, her two-day-old baby lay in an incubator in the neonatal intensive care unit. Jimmy was in his first month of a pediatric genetics residency; he and his attending physician had been called in for a consult. Jane had known there was a problem even before her newborn was delivered. An abnormality was found during a non-routine thirty-week ultrasound, replacing the young mother-to-be’s jitters with serious concern.
Neonatal Intensive Care Unit
Typically, CDH is spontaneous, meaning not hereditary. But Jane’s case struck Jimmy as unusual, and he carefully took down every detail she recalled. Her family medical history included a peculiar number of rare conditions, and Jane herself also had a developmental condition: an absence of the pericardium, the membranous sac that lines the heart.
Because both the pericardium and the diaphragm arise from the same underlying tissue, the young resident suspected that the absence of Jane’s pericardium and her baby’s CDH were related. He wanted to know all he could about Jane’s diagnosis in the 1980s. Fortunately, surgeons in the same children’s hospital where Jimmy was training had discovered and documented Jane’s condition and treatment three decades earlier. Jimmy unearthed her records from the medical archives. The thirty-year-old notes were revealing: during an operation to repair a common congenital heart defect, the surgeon wrote, “We suddenly found ourselves in direct communication with the heart.” The surgeon had commissioned an artist to draw the unusual defect. The image was reproduced on microfilm—a perfect representation of Jane’s pre-surgical heart.
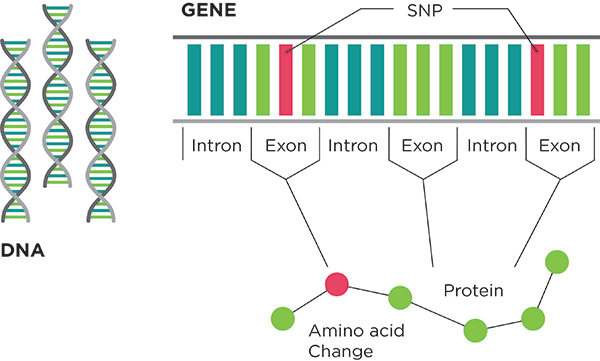
Exome Sequencing
A few months later, Jimmy attended the David W. Smith Workshop on Malformations and Morphogenesis. Held in a different city every year, this annual international meeting of doctors who specialize in birth defects is perhaps the most intimate professional event in clinical genetics; only sixty or so individuals are invited to participate, based on their submitted abstracts describing their work. Jimmy had the opportunity to present Jane’s unique case to his colleagues and get their feedback, all in less than fifteen minutes. His colleagues suggested he try using a relatively new technique called exome sequencing.
Exome sequencing is a refined version of whole genome sequencing. Rather than sequencing all of a person’s DNA, exome sequencing looks only at the 1% of a person’s DNA that codes for genes. The exome is a small slice of the whole genome, but it is perhaps the most important slice because it provides instructions for making all the proteins in your body. Perhaps Jimmy would find the key to Jane’s family’s genetic abnormalities there.
Chapter 5
Both exome and whole genome sequencing are powerful new tools for identifying the yet unexplained causes of rare genetic conditions. But they also bring with them new questions for patients, as well as for physicians and researchers; unfortunately, Jimmy’s dilemma was far from unique.
Exome and whole genome sequencing bring with them new questions for patients, as well as for physicians and researchers.
Two months before learning Jane was pregnant for the third time, he attended a talk by Dr. Heidi Rehm, the director of the Laboratory for Molecular Medicine at Partners HealthCare. Dr. Rehm was a guest speaker at Jimmy’s institution’s weekly seminar in genetic medicine. The windowless room was packed with doctors and researchers waiting to hear her presentation about the opportunities and challenges of genomic medicine. Most of her talk was dedicated to an explanation of an Internet-based system for managing patient genetic information in an era of personalized medicine; she led the team that developed a program called GeneInsight, which was tested and adopted by medical centers throughout the United States.

Heidi Rehm. Image Courtesy of Slone Partners.
One of the greatest challenges facing molecular diagnostic testing labs is the need to track information about an increasingly large number of genetic variants. Tracking incorporates genetic variants into test reports and keeps clinicians up to date on new discoveries. Doctors also need to track which genetic variants have been identified in each of their patients and what each signifies in terms of patient health. GeneInsight was intended as a solution for these challenges.
Rehm also spoke about the challenges of sifting through people’s genomes to figure out what results should be returned. To demonstrate this, she told a story about the kind of situation that’s every expectant parent’s nightmare.
The audience listened attentively as Rehm explained the situation: An anomaly was discovered in a prenatal ultrasound, and follow-up testing suggested a rare developmental genetic condition called Noonan syndrome. This condition affects many areas of the body, including the heart, bones, and facial features. Specifically, genetic testing of the fetus identified a mutation in the PTPN11 gene, which was known to cause Noonan.
But Rehm dug deeper. She explained that another lab had studied this particular mutation in PTPN11 and had discovered that the mutation was more common than first reported, and thus could not be the cause of an exceedingly rare condition. If the mutation did cause Noonan, then logically, many more people in the world would have the condition. Upon learning this information, Rehm checked her own database and ultimately ruled out the fetus’s specific mutation as causing Noonan.
The climax of Rehm’s story: the couple ended the pregnancy, presumably because of the PTPN11 mutation result. Murmurs of concern immediately resonated around the room, and a woman in the front row asked, “Was the family contacted?” Had the family been informed their mutation wouldn’t cause Noonan? Rehm didn’t know. She was the lab director, not the family’s physician.
Even when we are confident about genetic information, results can be uncertain; as Rehm’s story illustrates, such uncertainty can have real-life consequences. Although her lab had the ability to track this information in a database so as to correct the initial mistaken interpretation, this thoroughness is not yet the norm. This type of problem may occur more often as whole genome sequencing becomes commonplace. Even when accurate information is available, making sure it’s communicated in a timely manner is a significant challenge. In recognition of this problem, the federal government has passed a new rule that grants patients access to their lab results, bypassing their physicians. It isn’t clear whether this rule will apply to genetic tests.
Reflecting on the story later, Jimmy realized it resonated with his situation; he was afraid of exactly this sort of misinterpretation when deciding whether or not to disclose Jane’s result. He knew a false positive could result in the termination of a healthy and much-wanted child.
Chapter 6

Image Courtesy of digital cat via Flickr
As genetic testing becomes more prevalent, standards and best practices are needed. Rehm’s story is just one example of some of the challenges posed by new technologies. Exome and genome sequencing have immense potential in the diagnosis of rare genetic conditions and will undoubtedly impact increasing numbers of reproductive decisions. Yet, this test is like no other because it yields a very large volume of data. As a result, one of the biggest debates in the medical community at the moment is what should be done with all the other genetic findings that are produced.
There are both potential benefits and potential risks to revealing secondary or incidental findings that are unanticipated by the patient. On the one hand, patients may learn about their risks for conditions that could be prevented or treated with early medical intervention. They might learn about their risks for conditions late in life that they might want to plan for. On the other hand, learning these risks might also increase healthcare costs as patients will require more testing, at the very least to confirm the result. Patients will likely also require follow-up sessions, new specialist consultants, and extra diagnostic investigations. Finally, unanticipated information might cause unnecessary worry.
Patients may argue they have the right not to know.
Not surprisingly, doctors and patients sometimes disagree about how to navigate these risks. Clinical geneticists often want to protect patients and the public from the potential harms of genetic information, and typically take a cautious approach to returning results. This seems particularly reasonable when the case involves telling someone he or she has a fatal genetic condition, such as Huntington’s disease. Such a diagnosis can be devastating; even most individuals with family histories of such conditions choose not to learn whether they have them. Such patients may argue they have the right not to know. These individuals argue that patients would rather not know about their genetic information if the results are potentially uncertain and there are no treatment options.
Yet, patient preferences vary substantially; some patients demand results, arguing they have the right to know—a reasonable argument given recent efforts in healthcare to become more patient-centric and to value shared decision-making. This is clearly the case with genetic information that offers potential prophylactic treatment options—consider Angelina Jolie’s decision to undergo an elective double mastectomy after undergoing genetic testing for hereditary breast cancer. Yet, even when options are limited—for example, in the case of Alzheimer’s disease, where there is no certain test—many seek to plan for their future and their family’s future with as much information as possible.
Who should decide what results you get back? What should the guidelines be?
Regardless of where one sits along this spectrum, however, the burning question remains: who should decide what results you get back? What should the guidelines be?
As it happens, in the case of clinical whole genome and exome sequencing, there are recommendations, which were developed by the American College of Medical Genetics and Genomics (ACMG). In 2011, the ACMG put together a working group of clinical geneticists, genetic counselors, and others in order to compile a list of recommendations for clinical laboratories. They revealed this eagerly awaited document in March of 2013. In the recommendations, the ACMG advised that laboratories should report the results from analysis of fifty-seven specific genes to the physician who ordered the whole genome or exome sequencing. It would then be up to the physician to decide, in consultation with his or her patient, what results to disclose. The recommended fifty-seven genes are informative for twenty-four conditions, including many heritable, treatable cancers; several rare cardiovascular diseases; and one potentially fatal adverse reaction to anesthesia.
Image Courtesy of Daquella manera via Flickr
However, instead of giving the medical community a common consensus about secondary findings, the recommendations have led to more debate. Expert commentators remain divided on a number of issues. A major problem with these recommendations was that patients who opt for clinical sequencing in effect give up their ability to make further decisions; they no longer have the right not to know. This issue was recently addressed, in part, by the ACMG revising its recommendations to allow patients to “opt out” of receiving any secondary findings. The issue remains whether or not patients should be able to receive selected results from a menu of secondary findings.
Other researchers expressed surprise, comparing the ACMG recommendations to those recently released on pediatric genetic testing by the American Academy of Pediatricians (AAP). The AAP guidelines recommend that children should not receive genetic testing for adult onset conditions, but most of the twenty-four conditions on the ACMG recommended disclosure list fit this description. Further, many were dissatisfied by the ACMG working group’s argument that the benefits of returning a child’s test results to his or her family would be of benefit to other members of the child’s family. The authors of the recommendations argued that family members could now seek treatment for a disease they would not have been aware of. But although giving family members this information may be a benefit, returning results may not be in the best interest of the child, who, as an adult, might rather not know this information.
Quibbling over some of the details aside, this list has been published with the clout of the ACMG behind it, and now the question is: who will follow it? Currently, practices about returning secondary results vary among clinical laboratories. There is general consensus that the majority of labs that conduct exome sequencing will get in line with the recommendations, though it may take some time before there’s universal compliance across the United States.
And then, what will doctors do? Will doctors and their genetic counselors, who are responsible for counseling patients pre- and post-test for both main and secondary results, follow these recommendations? It is too early to know the outcome; even the authors of the ACMG recommendations expect that the guidelines will change rapidly and that practices will evolve as the medical community gains experience in returning exome and whole genome results.
Chapter 7
When Jimmy first met Jane, he had never analyzed a genome. He didn’t know how to use the specialized software, so he took a course on exome sequencing, during which he learned about the available tools and how to use them to fish for a mutation in a sea of A’s, T’s, C’s, and G’s. After practicing with two exomes and convincing himself he knew what he was doing, Jimmy decided he was ready to analyze the exome data he had received on Jane and her family.
To increase his chances of finding the cause of CDH in Jane’s family, Jimmy took genetic samples from six individuals: Jane, her husband, and her parents, as well as Jane’s first baby and the amniotic fluid from her second pregnancy. He sent the samples away for sequencing; the results arrived in the form of a large Excel file that had 90 thousand lines. Each of the six exomes contained about 20 thousand DNA variants, places where one letter of DNA varied from what was expected; many of these were shared within the family. Variants are changes in the base pairs of DNA, which differ from the general population; most are harmless, but some rare variants result in diseases such as CDH. As Jimmy sat in front of his computer screen, facing so many possibilities, he came up with several lists of variants that might possibly explain CDH in Jane’s family. His theory was that Jane had a de novo mutation, one which was not present in either of Jane’s non-symptomatic parents, but which arose uniquely within her cells. These de novo variants sat at the top of his list of potential candidates.
Jimmy worked on his analysis through the summer of 2012 in a communal workspace in his mentor’s lab. It took him almost a month. As a trainee in developmental genomics, he had several projects involving zebrafish that filled up his day. But at the end of a normal workday, the summer sun still hung high, fueling Jimmy’s inquiry, and after a quick bite of dinner, he sat down to analyze these exomes almost as a sort of hobby—something he was doing on his own watch.
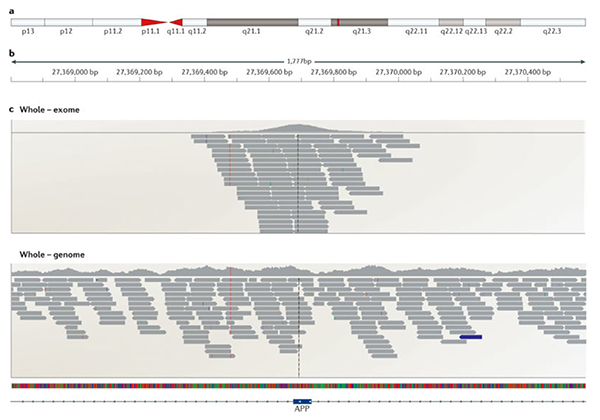
A portion of a gene comparing exome (top) versus genome sequencing (bottom). Image Courtesy of Nature Reviews Neuroscience.
One night, he found a de novo mutation in Jane’s genome in a known gene. The mutation was also present in the samples from her first baby and second pregnancy. Mutations in this gene were known to cause a rare absence of the pancreas, but had not been previously linked to CDH. This particular mutation (a stop-gain mutation that would lead to a truncated or shortened protein) was in an important coding region that would likely affect the resulting protein’s function.
The next day, he approached his colleague Gemma, a post-doc from South Africa, who had taught Jimmy how to analyze exome data.
“What do you think of this? It’s a stop-gain!”
The excel file was open on Gemma’s computer.
She peered at it for a moment. “If that is right, it would be the biggest beginner’s luck of all time! I’ll eat my hat if it validates.”
Not being entirely confident, Jimmy casually downplayed the finding and brushed off this comment with a laugh. “I’m sure it’s not right.”
Despite the requisite humility, his gut was telling him the opposite. This was his top candidate by far, with fifty other genes tied for second place. It was time to send the samples off to test the gene for this mutation using Sanger sequencing, a tried and tested sequencing technique that has been in use since the late 1970s. Sanger sequencing involves making lots of copies of a template DNA, each ending at a different point of the sequence. In the process, each length of DNA is fluorescently tagged. When spread out by length, the DNA sequences separate, allowing one to read each consecutive letter of the sequence. Having been replaced by faster Next-Gen methods for large-scale projects such as exome and whole genome sequencing, Sanger is still used to analyze smaller sections of DNA and to validate mutations found by Next-Gen sequencing due to its high accuracy.
A few days later, having received the Sanger results, Jimmy went to his lab. It was located on the tenth floor of a modern downtown biotech building, which had been purchased by the children’s hospital when the economy took a dive. He needed the genetic software on a certain computer to conduct his analysis. It was nighttime again; everyone else had already left for home. Sitting at the stainless steel bench, he looked at the screen.
“Holy shit! It’s here—in the stop. The letters change from G to T.”
He saw it in Jane. It wasn’t in either of her parents. He had found it. He had been working as both a researcher and a doctor for a number of years, but this moment was different.
Jimmy remembers thinking: This is the best and most important discovery I have made to date.
Staring at the computer, he double- and triple-checked to make sure he was right. There was no one around to share his excitement. He stood up and moved to the adjacent lab bench. Readying the necessary materials, he began preparing a new set of samples to sequence and validate just to make sure. When he eventually arrived home on his bike, he rushed in to tell his wife the news.
Chapter 8
Jimmy now had a potential answer for Jane. As it turned out, she had some news of her own: she was pregnant again! Unfortunately, although Jane was confident of her test results, Jimmy could not yet be certain of his. Before he could reveal anything to her, he needed to independently validate his findings, search for similar cases, and get permission from the IRB. And then—even if this all went in his favor—he had to decide whether to share the result with Jane at all.
Image Courtesy of surlygirl via Flickr
All things considered, though, Jimmy’s timing was perfect. Not only did he have this result in hand, but he also found, in the scientific literature, a case of CDH where the affected individual had both a congenital heart defect and a mutation in the same gene. On Thanksgiving, he finally got the validation results back, and they confirmed the accuracy of his research results. The mutation was definitely present in Jane, and it was also associated with the correct diseases. Jimmy’s confidence grew. He suspected Jane would want to know—so he filed paperwork with the IRB. He contemplated the potential bad outcomes of his decision; most of all, Jimmy was very worried this information would lead to terminating a healthy fetus if Jane decided to end the pregnancy in the next month, before the fetus had developed enough to visually confirm the presence or absence of CDH by ultrasound. On the other hand, even without testing the fetus for the mutation, she could decide to terminate at any time. She could wake up one morning and think, Oh my god, 50% risk, and decide it was too great. In that case, no one would ever know whether the child was developing CDH.
Even so, Jimmy’s gut told him Jane should know everything he knew. He hoped the IRB would see it the same way.
The morning after Jimmy’s call with Doug, an email from the IRB arrived in Jimmy’s inbox. Finally, he had their decision, and within a few minutes of receiving the email, he picked up the phone and called Jane. He apologized for not being able to contact her the day before and told her he had just received approval to speak with her. He asked her whether it was a good time to talk. He did not want to leave her hanging, waiting for days before making the long journey back to the clinic. She was anxious to hear Jimmy’s news. He told her everything he had found and explained that he might have an answer as to the cause of CDH in her fetuses. He also explained that this information could be used as the basis for a prenatal test called chorionic villus sampling (CVS). CVS is a procedure to acquire fetal DNA for genetic testing; it can be conducted either transvaginally through the cervix or transabdominally, similar to an amniocentesis test.
Jane was eager to know more, and excited: “Now I have something I can do!”
Jimmy was relieved when Jane confirmed his decision to offer her the information. “Exactly,” she agreed. “If you hadn’t told me, I would have been angry.” The Jimmy-Robbie rule had worked.
Jimmy advised Jane and her husband to make an appointment to see him and their genetic counselor, to talk about the result and their options in person, and the following Monday, she made the long journey back to the hospital. Jimmy wanted her to have the results of the test before her eighteen-week ultrasound; he knew making a decision to terminate at fifteen versus nineteen weeks is significantly different.
That Monday, Jimmy met with Jane, her husband, and her OB/GYN team to review Jimmy’s findings and offer her the opportunity to screen her fetus for the mutation. In the small consultation room, Jimmy showed them the handout he had spent hours putting together the night before. He explained the risks and recommended they continue with their eighteen-week ultrasound even if the test suggested a healthy fetus, unaffected by CDH. He also explained that even if the fetus was found to have the mutation, there was still some uncertainty because it was a research result: “There’s still a possibility you could be terminating a healthy fetus.”

Image Courtesy of corncob82 via deviantart
Despite Jimmy’s warnings, Jane had made her decision to test the fetus and had no use for the tissues strategically placed in the center of the table in the prenatal genetic counseling room. She asked to do the CVS immediately. Her prenatal team was surprised, but they fit her in later that day. No mutation was found.
The baby was born in June, healthy as could be. Jimmy was very happy when Jane sent him a picture of her son.


